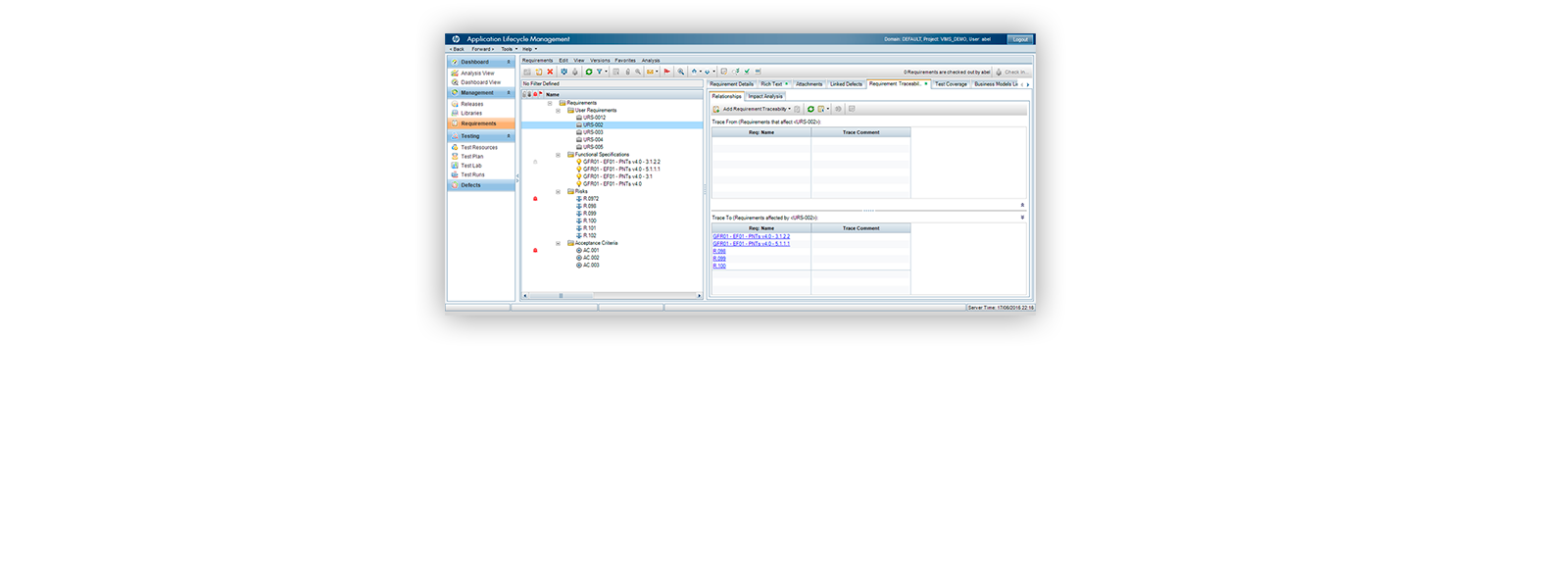
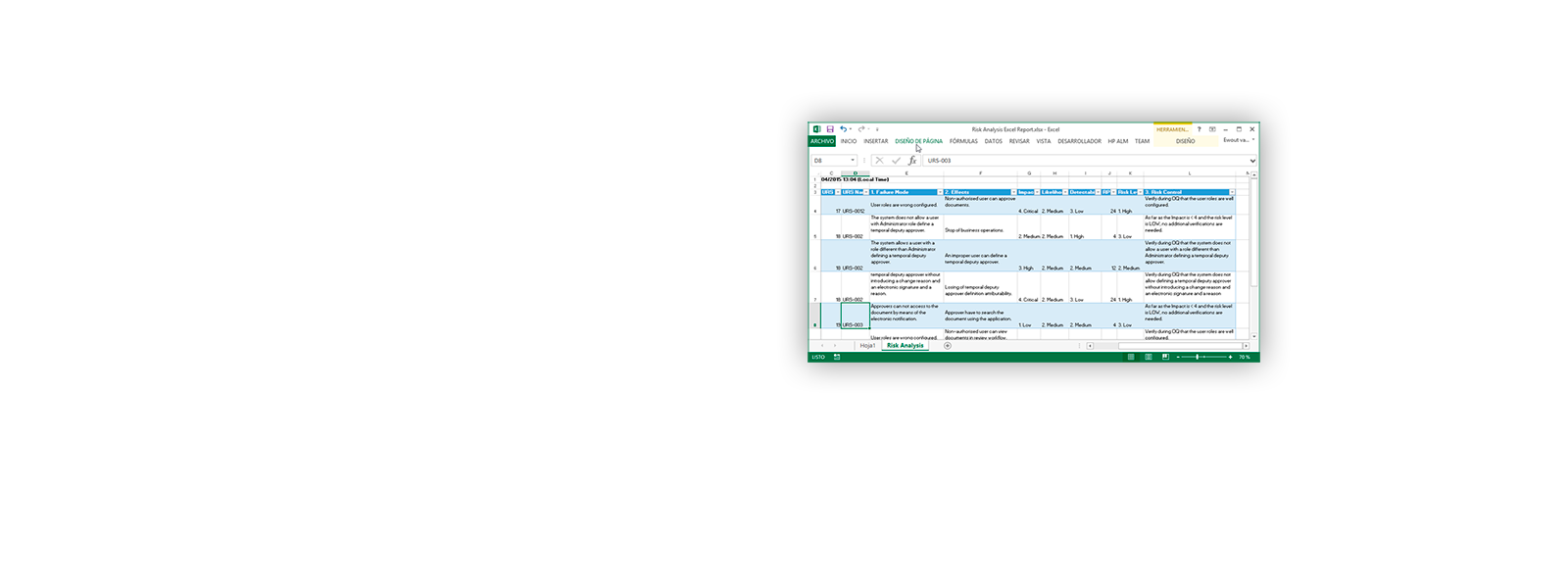
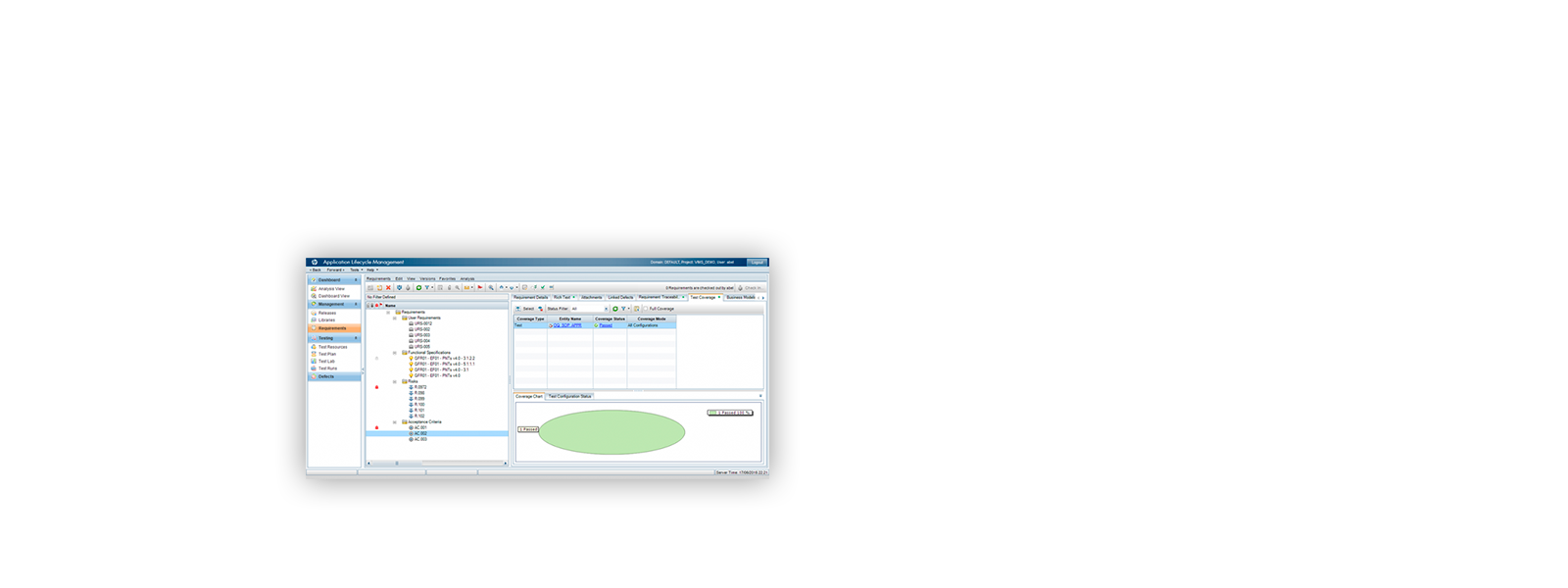
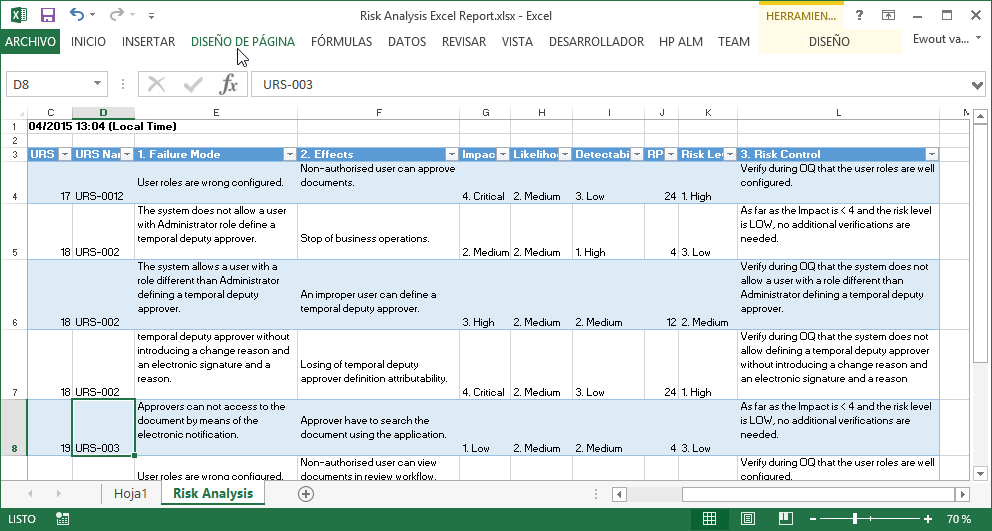
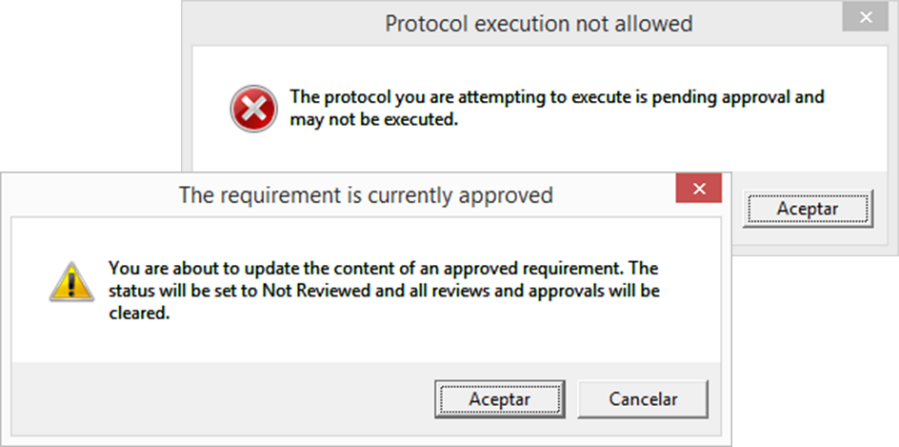
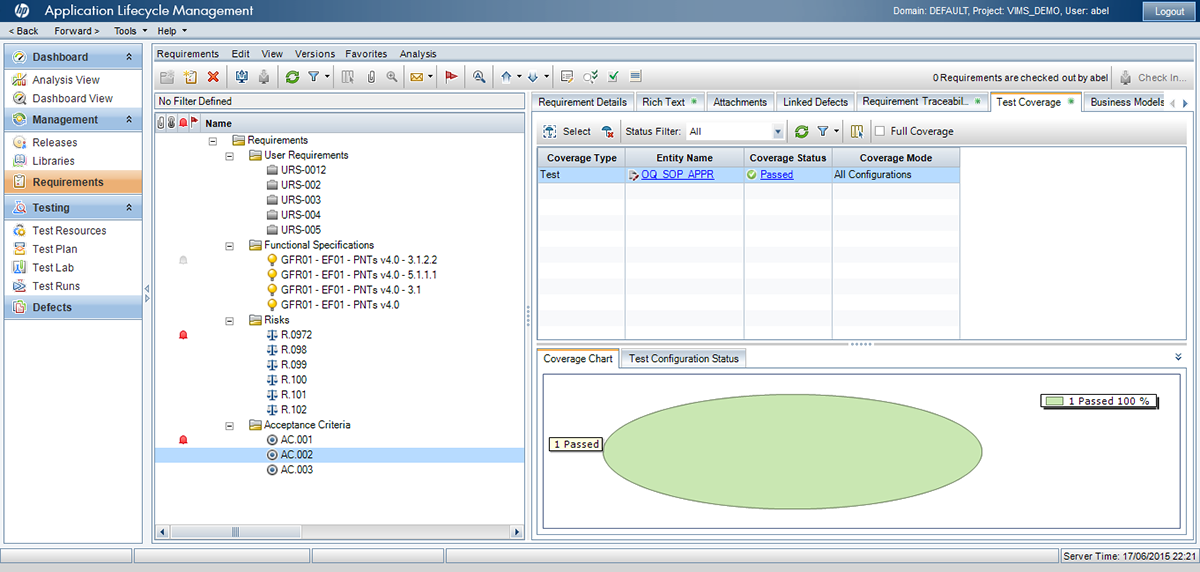
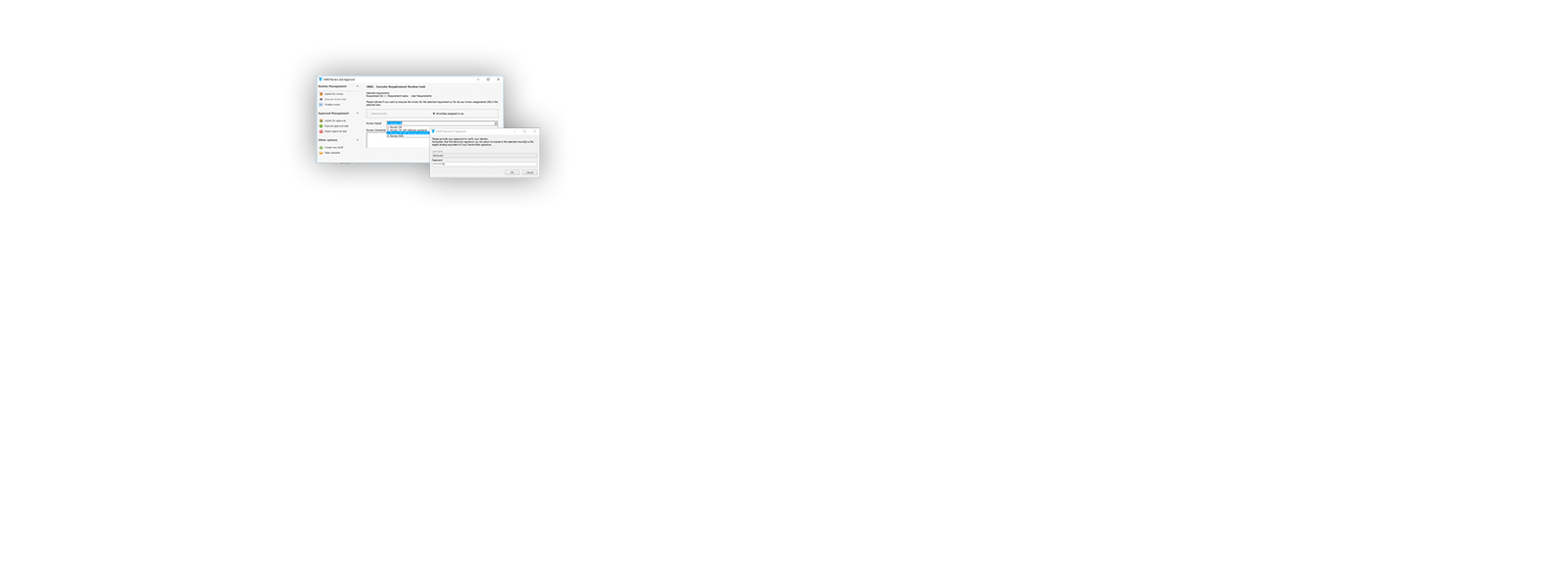¡ViMS es la aplicación de referencia del sector de ciencias de la vida porque garantiza el 100% del cumplimiento del entorno regulador en sus procesos de validación!
Qualitate es una consultora especializada en el sector de ciencias de la vida que desarrolla ViMS – Validation information Management System.
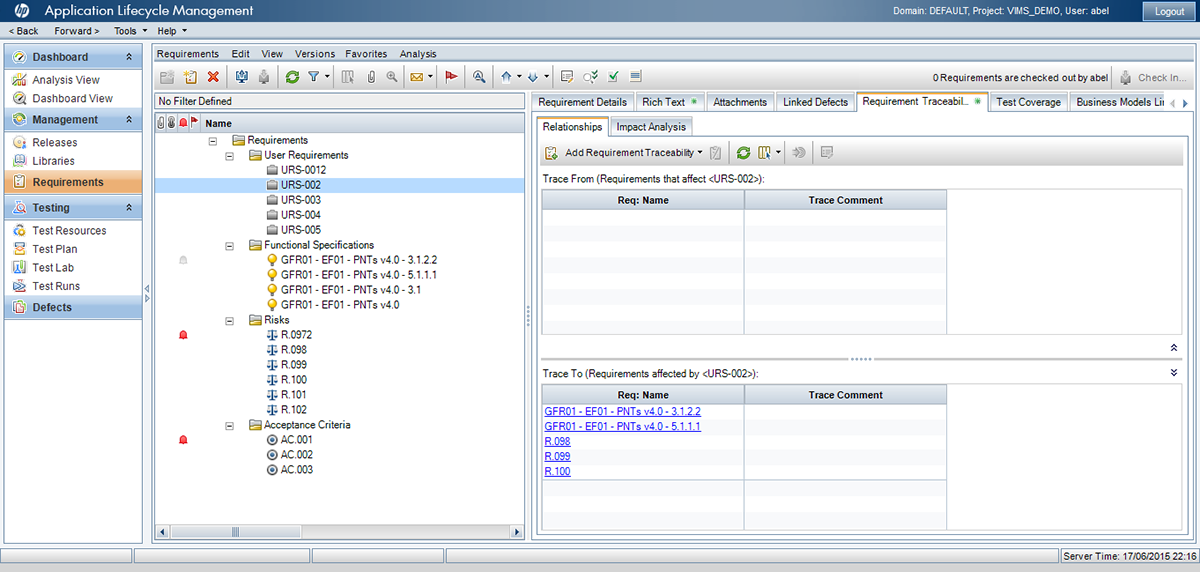
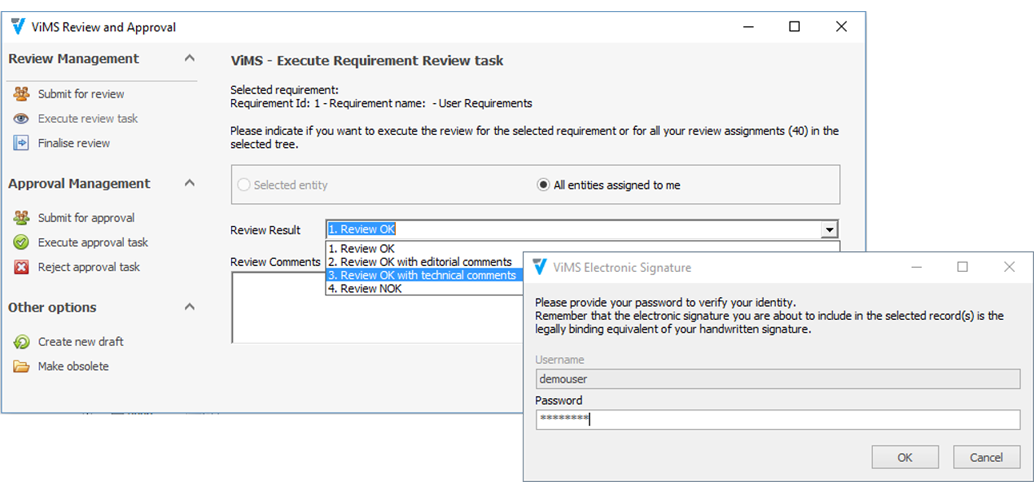
Utilizando las aplicaciones de gestión del ciclo de vida de nuestros socios tecnológicos Micro Focus – Hewlett Packard Enterprise, y con el asesoramiento de consultoras especializadas en el sector de ciencias de la vida, Qualitate garantiza que sus procesos de validación se adaptan al marco legal, como 21 CFR Parte 11, EU-GMP Anexo 11 y GAMP.